
Additionally, different reactants may be in different phases of matter at different ambient temperatures, which can also play a role in heat transfer. The same reactants in a reaction can vary in the amount of heat they can transfer when at different temperatures. Temperature DependenceĪ factor that comes into play when determining enthalpy, even though it is not seen in the equations above, is temperature. However, sometimes you may see the units of calorie or British thermal unit (BTU). The SI unit of measurement for enthalpy is the Joule (J). You also may see pV expressed as W, known as work. This property is the sum of a system’s internal energy and the product of Pressure x Volume. This is due to the law of energy conservation. This is the change in internal energy that is equal to the heat transfer within the system. This means that enthalpy depends only on the final energy, pressure, and volume and not the path the system took to get to the final state. Topics Covered in Other ArticlesĮnthalpy (H) has to do with thermodynamics it is a state function, at constant pressure, used in chemical and biological systems. In addition, you will learn about some of its applications, as it relates to thermodynamics. Heike Kamerlingh Onnes coined the term “enthalpy” while Clausius coined the term “entropy.In this tutorial, you will learn about the definition and equation of enthalpy. “Enthalpy” is the transfer of energy while “entropy” is the Law of Disorder.Įnthalpy takes the “H” symbol while entropy takes the “S” symbol. Clausius was the one who coined the term “entropy.” The symbol for entropy is “S” which states that the world was viewed to be inherently active wherein it acts spontaneously to scatter or minimize the presence of a thermodynamic force. Carnot stated that thermodynamics is the flow of heat from higher to lower temperatures that makes a steam engine work. Clausius and Thomson were inspired by Carnot’s observation of a stream that makes the turning of a mill wheel. In the middle of the last century, “entropy” was already formulated with the extensive efforts of Clausius and Thomson. It is essential in understanding life and cognition. It is one of the most fundamental laws in the field of physics. “Entropy” is the second law of thermodynamics. In other words, heat may be absorbed or released by a certain chemical reaction under such conditions.
ENTHALPY VS ENTROPY PLUS
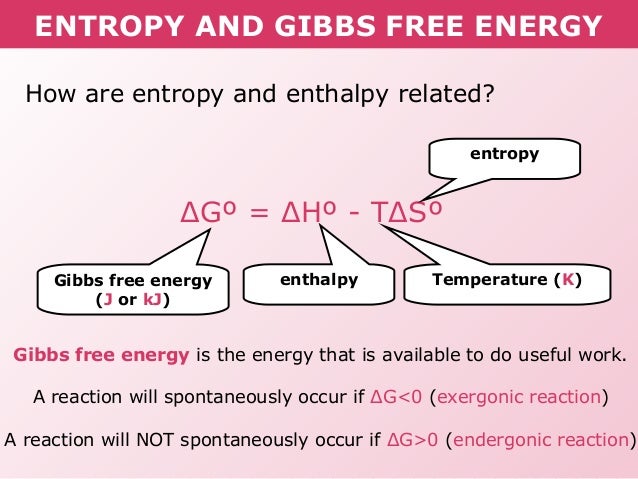
Under constant pressure, enthalpy is equivalent to the change of the system’s internal energy plus the work that the system has exhibited to its surroundings. To put it simply, the enthalpy of a system is equivalent to the summation of non-mechanical work done and the heat supplied. In endothermic reactions, there is a positive change in enthalpy, while in exothermic reactions, there is a negative change in enthalpy. Only the change in enthalpy is the preferred measurement of quantity rather than the enthalpy’s absolute value. It is impossible to achieve the value for the total enthalpy because a system’s total enthalpy cannot be directly measured. Porter was the one who designated the “H” symbol for “enthalpy.” In biological, chemical and physical measurements, enthalpy is the most preferred expression for system energy changes because it has the ability to simplify particular definitions of energy transfer. Heike Kamerlingh Onnes was the person who coined the word while Alfred W.
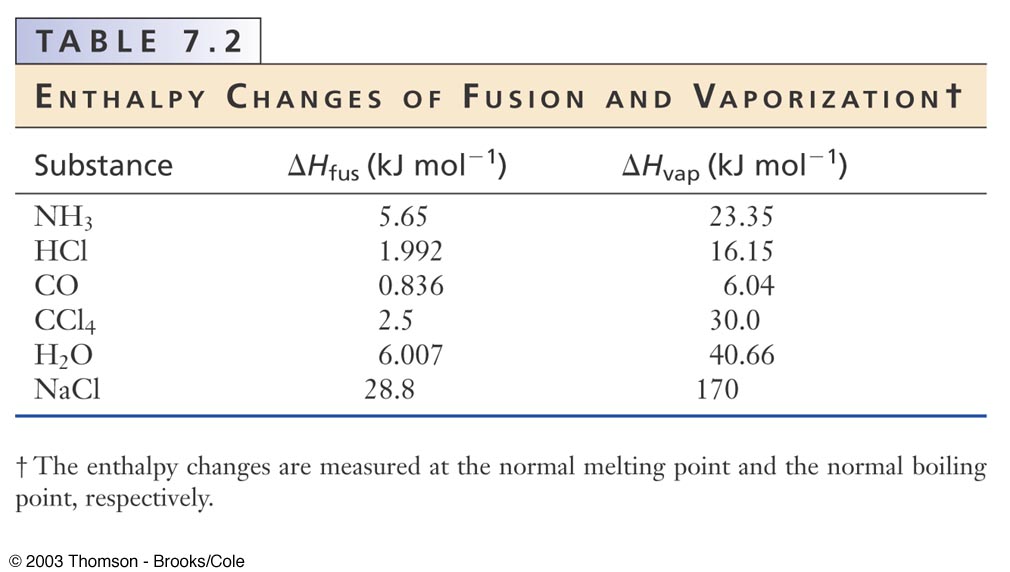
“Enthalpy” is from the Greek word enthalpos (to put heat into). The enthalpy’s unit of measurement is the joule (International System of Units) and the calorie (British Thermal Unit). This energy serves as the push or the trigger to build a system. To create a thermodynamic system, internal energy is required. In a thermodynamic system, the measure of its total energy is called enthalpy. In this article, you will learn more about the differences between enthalpy and entropy. The two most common principles of thermodynamics are enthalpy and entropy. As long as heat and friction are involved, there is thermodynamics. Applications of thermodynamics are exhibited in the flow of electricity and from just a simple twist and turn of a screw and other simple machines. It is a study concerned with the relationship of heat to different forms of energy and work. “Thermodynamics” is a branch of natural science which involves the study of the body systems’ internal motions. Our modern world today is integrated with the laws of science such as thermodynamics. Biologists, chemists, and physicists are only a few people who try to seek for answers. These are everyday phenomenon that we encounter in our lives, but those people who are not inquisitive enough never attempt to look for answers why such phenomena exist.
One human stares at the ground and wonders how plants are able to grow. One human looks at the sky and wonders how the rain is formed. Curiosity is one aspect of a human which helps him discover the different phenomena in the world.


 0 kommentar(er)
0 kommentar(er)
